Review the most up to date literature published and speculations/anecdotes and cited articles from around the globe.

Speculations:
- Pulmonary edema and infiltrates are being caused by coronavirus binding of pulmonary ACE, causing capillary leak and upregulation of ACE-2.
- Based on the sequence similarities of the RBM between SARS-CoV-2 and SARS-CoV, several independent research groups investigated if SARS-CoV-2 also utilizes ACE-2 as a cellular entry receptor.
- anti-ACE-2 antibodies or could be used to block SARS-CoV-2 binding to the receptor. Additionally, TMPRSS2 inhibitors could be used to prevent SARS-CoV-2 entry into host cells. Camostat has been used to treat chronic pancreatitis in Japan and is currently undergoing Phase 1/2 trial testing in the United States.26 If deemed safe, camostat could be a potential treatment option of CoV infections.27 It is also possible that antibodies developed during SARS-CoV infection could help prevent or treat COVID-19. Hoffmann et al. showed that sera from recovering SARS patients reduced SARS-CoV-2 S protein-driven entry into Vero-E6 cells.21 However, future studies are needed to determine whether any of these options are effective in disrupting the interaction between virus and receptor in vivo.
https://www.rndsystems.com/resources/articles/ace-2-sars-receptor-identified
Clinical presentations and concerns
- Acute respiratory distress syndrome (ARDS) is a major complication in patients with severe disease. In the study of 138 patients ARDS developed in 20 percent after a median of eight days, and mechanical ventilation was implemented in 12.3 percent https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19/abstract/39 ]. In another study of 201 hospitalized patients with COVID-19 in Wuhan, 41 percent developed ARDS; age greater than 65 years, diabetes mellitus, and hypertension were each associated with ARDS https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19/abstract/57
- Some information from Europe is that there appears to be severe myocarditis and/or severely depressed LVEF <10% occurring in some patients with rapid decline around the 7-9 day mark after symptomatic infection.
- in the setting of severe cardiogenic shock, may suffer VT/VT or PEA and ultimately pass once this state is reached. In europe this presentation was more rare, however improved in some patients with IVIG and glucocorticoids.
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaa190/5807656
Investigational treatments
- no controlled data supporting the use of any of these agents, and their efficacy for COVID-19 is unknown (22).
- Remdesivir – Remdesivir is a novel nucleotide analogue that has activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro and related coronaviruses (including SARS and MERS-CoV) both in vitro and in animal studies (22).
- Chloroquine/hydroxychloroquine – Both chloroquine and hydroxychloroquine inhibit SARS-CoV-2 in vitro (22) .
- Lopinavir-ritonavir – protease inhibitor, for HIV infection, has in vitro activity against the SARS-CoV [30]
- Tocilizumab – Treatment guidelines from China’s National Health Commission include the IL-6 inhibitor tocilizumab for patients with severe COVID-19 and elevated IL-6 levels (31).
- Others include IVIG, steroids, oseltamivir, interferon beta and convalescent serum. No published data as of yet.
Other data:
- March, 2020, the WHO declared the COVID-19 officially a Pandemic.
- Per ACOG ” The approach to prevention, evaluation, diagnosis, and treatment of pregnant women with suspected COVID-19 should be similar to that in nonpregnant individuals”
- (ACOG) specifies that infants born to mothers with confirmed COVID-19 should be considered a patient under investigation and appropriately isolated and evaluated (32).
EMRAP link to LIVE and ongoing COVID updates
https://covid.emrap.org/
Centers for Disease Control and Prevention (CDC)
- Coronavirus (COVID-19) Updates
- Resources for Healthcare Professionals
- Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19)
- Travel Guidance
- Situation Report
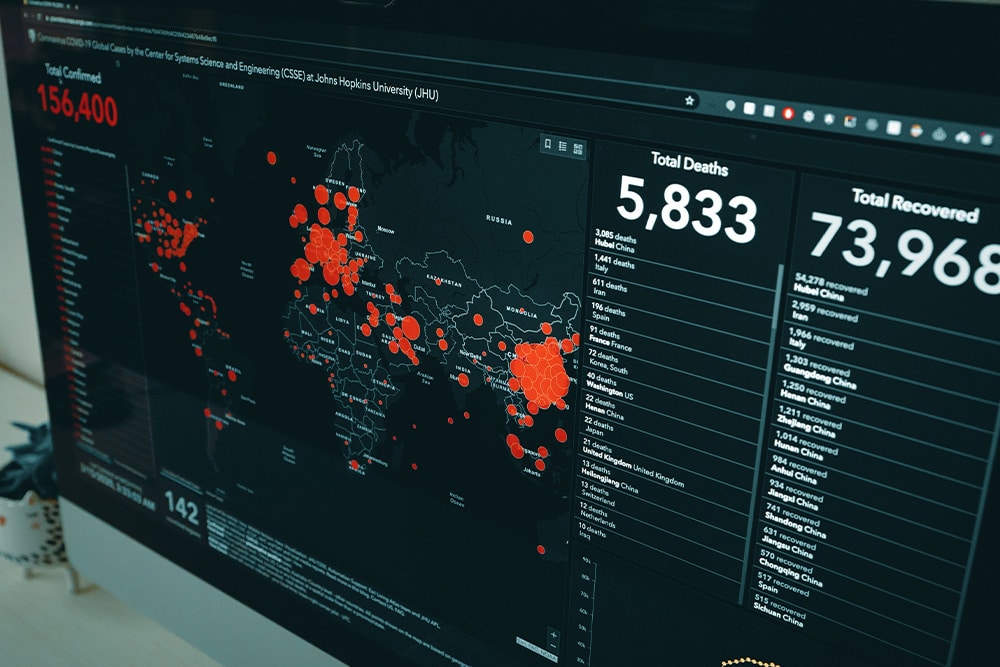
Real time link to the Coronavirus map and current Published global cases:
References:
- Fehr, A.R. and S. Perlman (2015) Methods Mol. Biol. 1282:1.
- Centers for Disease Control and Prevention (n.d.) Common Human Coronaviruses. https://www.cdc.gov/coronavirus/general-information.html.
- Yang, Y. et al. (2020) J. Autoimmun. [Epub ahead of print].
- Gu, J. and C. Korteweg (2007) Am. J. Pathol. 170:1136.
- Zhou, P. et al. (2020) Nature [Epub ahead of print].
- Schoeman, D. and B.C. Fielding (2019) Virol. J. 16:69.
- de Wit, E. et al. (2016) Nat. Rev. Microbiol. 14:523.
- WHO (2003) Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.
- WHO (2020) Coronavirus Disease 2019 (COVID-10) Situation Report – 50.
- Lu, R. et al. (2020) Lancet. 395:565.
- Li, F. (2016) Annu. Rev. Virol. 3:237.
- Wan, Y. et al. (2020) J. Virol. [Epub ahead of print].
- Li, W. et al. (2003) Nature 426:450.
- Riordan, J.F. (2003) Genome Bio. 4:225.
- Kuba, K. et al. (2010) Pharmacol. Ther. 128:119.
- Jinag, F. et al. (2014) Nat. Rev. Cardiol. 11:413.
- Ksiazek, T.G. et al. (2003) N. Engl. J. Med. 348:1953.
- Harmer, D. et al. (2002) FEBS Lett. 532:107.
- Leung, W.K. et al. (2003) Gastroenterology 125:1011.
- Tikellis, C. and M.C. Thomas (2012) Int. J. Pept. 2012:256294.
- Hoffmann, M. et al. (2020) Cell [Epub ahead of print].
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. U.S.A. 106:5871.
- Matsuyama, S. et al. (2010) J. Virol. 84:12658.
- Iwata-Yoshikawa, N. et al. (2019) J. Virol. 93:e01815.
- Huang, L. et al. (2003) J. Biol. Chem. 278:15532.
- Ramsey, M.L. et al. (2019) Trials 20:501.
- Zhou, Y. et al. (2015) Antiviral Res. 116:76.
- https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19
- Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China JAMA 2020
- https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19/abstract/77
- Reuters. China approves use of Roche drug in battle against coronavirus complications. https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF (Accessed on March 11, 2020).
- American College of Obstetricians and Gynecologists. Practive Advisory: Novel Coronavirus 2019 (COVID-2019). https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019 (Accessed on February 26, 2020).
- Seattle: COVID-19 patients compromised by comorbidities https://www.auntminnie.com/index.aspx?sec=sup&sub=xra&pag=dis&ItemID=128508
Article Peer Reviewed By:
Anant Patel DO FACEP @AnantPatels


This Post Has 0 Comments