Case:
HPI: Patient 62 yo man PMH of CAD s/p cabg one month prior, Substance abuse, COPD, ESRD, afib, aortic aneurysm presents with acute altered mental status during dialysis. Patient was unable to give any medical history or speak as his mentation was so poor. Per medical record, patient had: Paroxysmal Atrial fibrillation on eliquis, End stage chronic kidney disease on dialysis, aortic root dilatation s/p CABG for triple vessel disease, CABG one month prior, chronic drug abuse history, diabetes.
V/PHX: Patient in no acute distress. Comfortable appearing. Arousable but could not speak appropriately, mumbling, GCS of 11. Vitals were BP was 120s/low 50’s HR 70’s RR high teens O2 saturations maintaining above 95%.Physical exam showed GCS 11 (m6e3v2) and a diastolic grade 3/6 murmur heard best over the aortic field. Lungs clear bilaterally, no JVD noted, no pitting edema, rest of exam without abnormal findings.
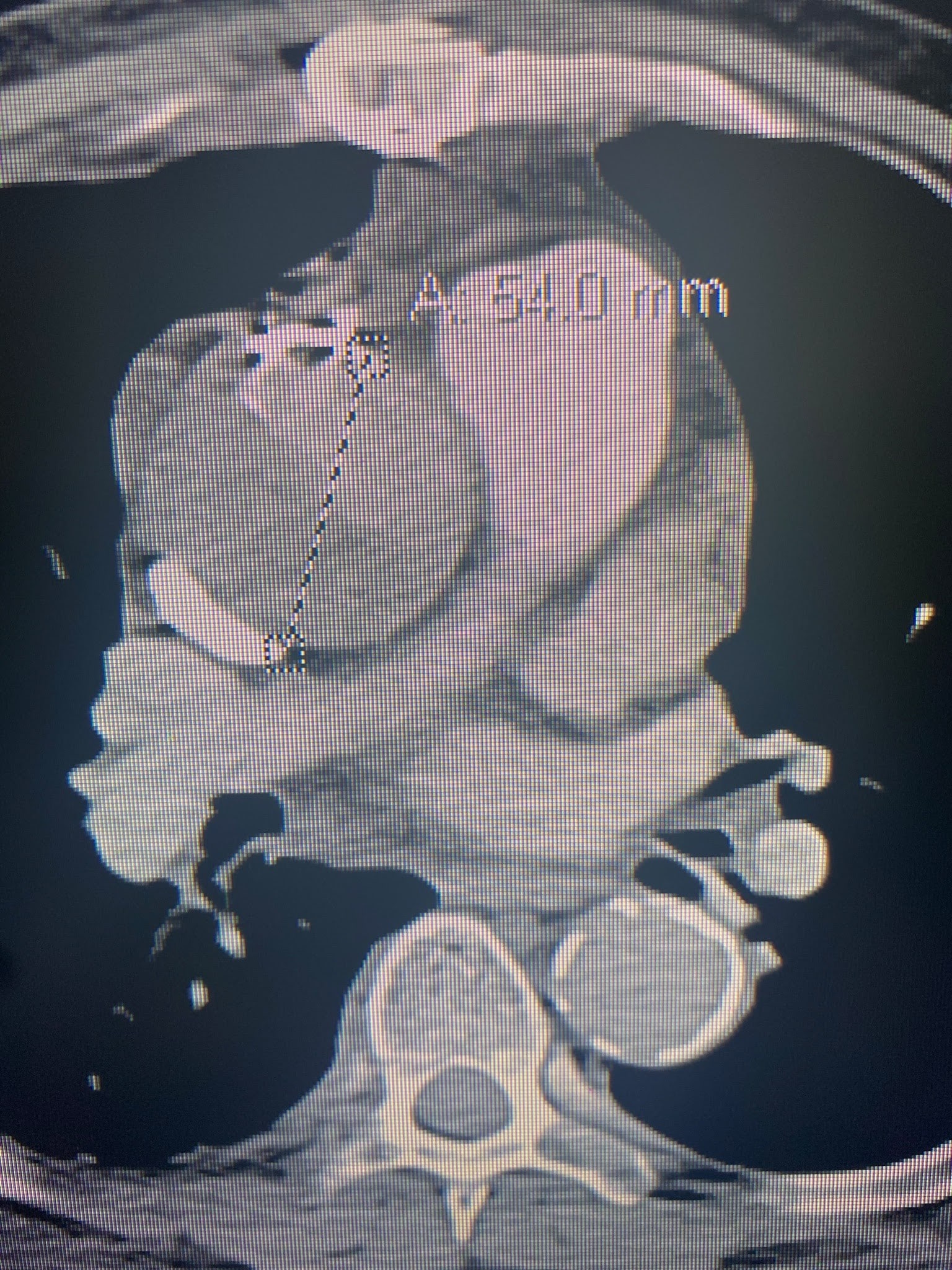
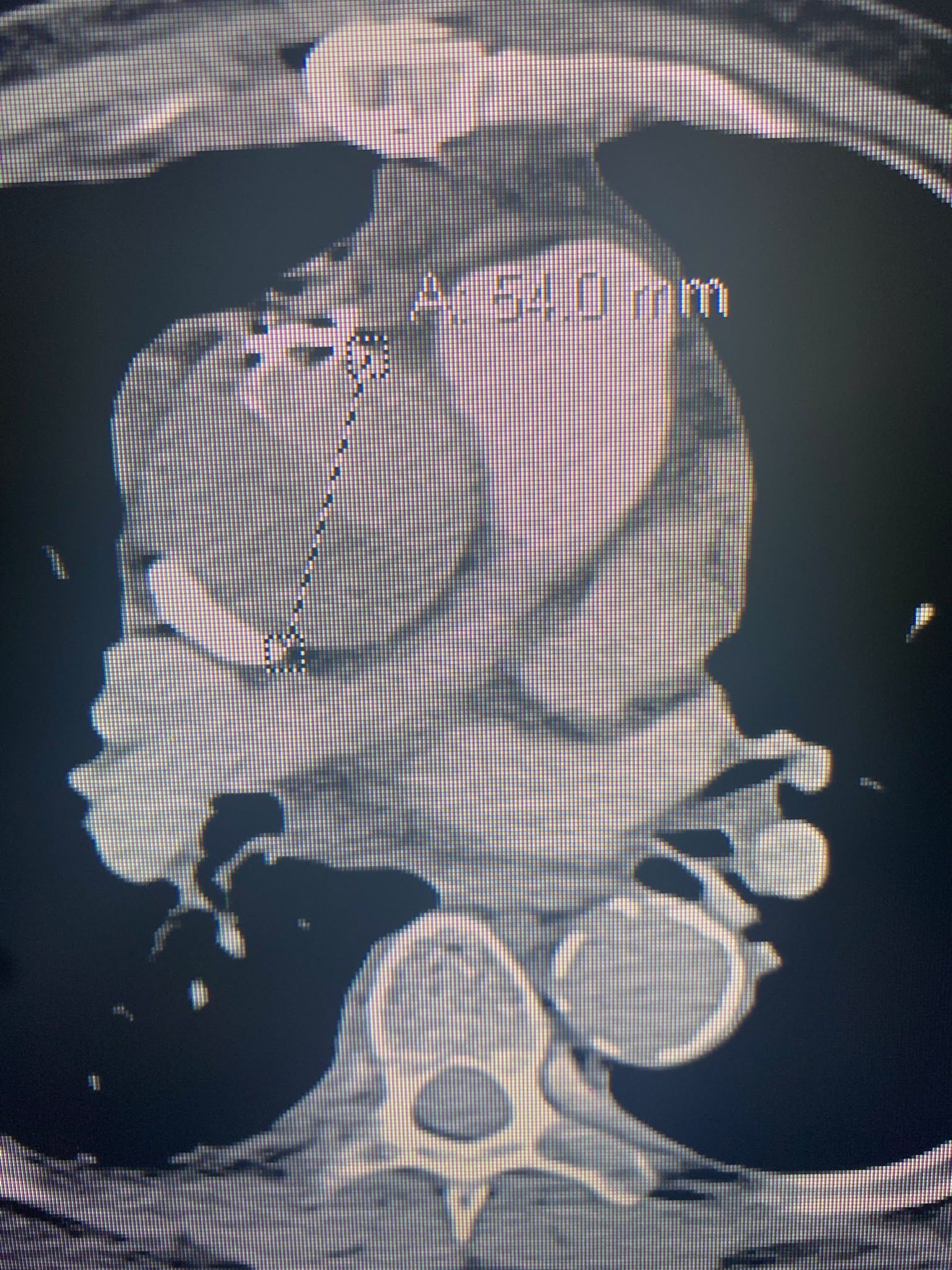
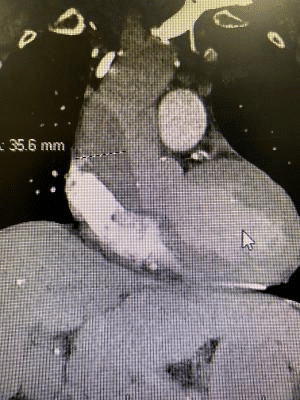
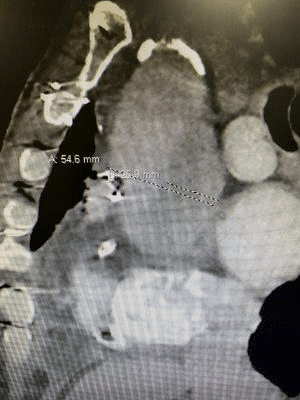
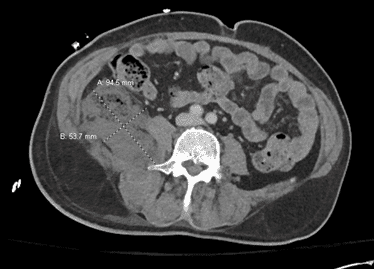
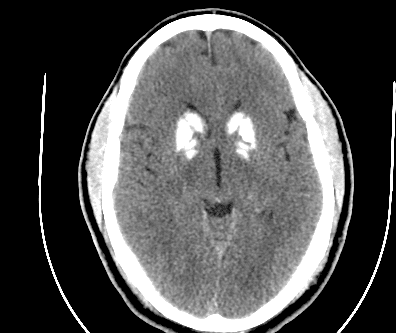
Labs and imaging: positive Lab evaluation showed a BNP of 175,000, Creatinine of 6, BUN 30, HGB 7.6, Troponin 0.048, XR negative for acute abnormalities, and a CT PE protocol with abdomen and pelvis with a new aortic root dissection without extension into any other arteries, seen in the next three pictures.
Clinical Course:
Patient started to have continuous bouts of hypotension. CT surgery was emergently consulted after CT read. After discussion with them, a bedside echocardiogram was done which showed new pericardial effusion without signs of tamponade. CT surgery elected to emergently take patient to the OR. Patient’s blood pressures continued to trend down, with late blood pressures ranging from 95-85 systolics over 40-50’s diastolic, leaving little medical room to work with. The patient was given PCC as adexanet alfa is not available at facility (5).
Blood was ordered and was ready at bedside, however we elected to hold it until he was in the OR. His blood pressure was soft, however the risk of giving the patient blood and possibility of rupture was found to outweigh the benefit of increasing the patient’s blood pressure. Patient was taken to the OR emergently where he had revision of two of his coronary artery bypass grafts as well as resection and graft replacement of his proximal aorta. Patient’s clinical course was complicated by a sternotomy infection, with an incomplete treatment course as the patient left AMA during his antibiotic treatment period.
Review of Thoracic Aortic Dissection:
Aortic dissection is a tear within the tunica intima into the media that creates a false lumen, and spreads between the intima and media layers dissecting them apart (1,3). Pathophysiologically, they occur at sites with the most sheer/hydraulic stress, similar to a river bend causing destruction of a river bank. The R lateral wall of the ascending aorta and the proximal segment of the descending aorta just beyond the L subclavian artery at the most common sites (1,3).
The cause of aortic dissections is thought to be similar to atherosclerosis, where you have chronic wall inflammation, leading to smooth muscle apoptosis degenerating the tunica media and elasticity of the aortic wall, which eventually ruptures and tears (1,3). Cellularly, macrophages invade the intima and release inflammatory cytokines and matrix metalloproteases which causes a cycle of collagen and muscular breakdown and poor repair (3).
There are two different ways to classify dissections: Debakey and Stanford. Debakey classification: Type 1 dissections make up 50% of dissections with the start point being in the ascending aorta with extension to the aortic arch, type 2 are 35% and are confined to the ascending aorta without involvement of the bachiocephalic or innominate, and 15% are type 3 which start in the descending aorta distal to the left subclavian (1,2,3). Stanford system: Type A involves the ascending aorta, type B does not (1,2,3).
Risk factors:
Atherosclerosis, hypertension, aneurysms (especially sacular), aortic valve stenosis, fluid overloaded states, dyslipidemia, aneurysm of the aorta, aneurysm elsewhere in the body, Marfan’s syndrome, Ehlers-danlos syndrome, Turner’s syndrome/bicuspid valve, Behcets disease, Giant cell arteritis, takayasu arteritis, infection in the aorta, blunt chest wall trauma/decelaration injuries (1,3). Any dilation of a thoracic aorta with a diameter over 50% of the normal is considered an aneurysm, putting them at high risk for dissections (4). This patient had a recent CABG and known aortic aneurysm, putting him at increased risk.
Symptomatically, it can cause any arterial injury from the heart to below. Proximally, it can dissect into coronary arteries, clot coronary arteries, dissect into the aortic valve causing acute regurgitation (as in my patient), dissection into the pericardial sac causing tamponade, rupture through the adventitial layer (leading to rapid exsanguination in the thorax) or claudication/acute thrombosis/blockage/inability for perfusion of the braciocephalic artery, subclavian, recurrent spinal artery causing paralysis from mid thorax down, carotids/cerebral arteries causing stroke, down to the SMA or IMA or Celiac causing bowel ischemia, renal artery obstruction, and more (1,2,3). Can also cause embolisms, with reentrance of the dissection clot back into circulation, causing ischemic limbs or arteries (1,3).
Typical symptoms in history are chest or back pain, focal claudication/arterial blockage causing weakness, renal failure, stomach pains, flank or back pains, and many more(1,2,3,6,8) The pain is described as excruciating chest, intrascapular, or precardial with radiation to other sites previously stated. Typical description is tearing. Associated symptoms include: Syncope (both cardiac with vasovagaling, baroreceptor activation, or neural syncope with acute cerebral artery obstruction), malperfusion symptoms (stated in previous paragraph) (1,2,3,6,8). Neurologically, they can present as ischemic stroke, spinal cord ischemia, neuropathy, hypoxic encephalopathy, syncope, and seizures, like this patient having encephalopathy and altered mental status (8).
Physical examination can be normal ranging to rapidly decompensating. Vitals can be normal or abnormal or unstable, with any range there. Wide pulse pressure from aortic regurgitation, decreased pulse pressure from tamponade, CHF causing flash pulmonary edema and hypoxia, etc. They can have: New heart aortic regurgitant (like my patient, diastolic decrescendo murmur ideally), distant/muffled heart sounds, JVD/CHF appearance, and any focal arterial blockage (6). Only 20% of patients will have a pulse deficit or differential between limbs (1).
Diagnostically, a CTA (95%< sensitive with a PPV of 100% and NPV of 86%), MRA (with 100% sensitivity), or TEE(97%-99% sensitive) are the highest specific and sensitive imaging (1,2,3). A chest XR has between 60-90% sensitivity for an enlarged mediastinal shadow. On CT, MRA, and TEE, you will be able to see a true and false lumen, confirming the diagnosis. However, TEE or TTE are required for dynamic testing of cardiac functioning and pericardial tamponade symptomatology. You can also use a d dimer for it’s high sensitivity, 97% sensitive, however specificity is lacking in evidence for (3).
Treatment:
Normal medical therapeutic interventions for either stanford type A or B dissections (type A involved the ascending arch, type b does not involve the ascending arch) are to maintain a systolic blood pressure under 110 with beta blockers, to decrease HR and shearing forces to decrease spread of the dissection (1,2). Addition of antihypertensive agents, like nitroprusside, are also a good additive however HR reduction for BP control is the primary goal for medical management (1,2). Ultimately surgical treatment is required for ascending aortic dissections, arterial occlusion, complete dissection, or when medical treatment failures (1,2).
Complications of an acute thoracic aortic dissection after open surgical repair include strokes, aneurysmal dilatation, surgical site infection (as seen in this case), sternal dislocation, and graft rupture (7). Endovascular repairs can lead to symptomatic endoleaks, dissection propagation, stroke, stent infection, stent erosion/aortoenteric fistula, stent migration, limb ischemia, and stent fracture (7). If you suspect a post thoracic dissection complication, resuscitate appropriately and consult your vascular surgeon as early as possible (7). Stable patients should receive a CTA, where unstable patients should receive a TEE for evaluation (7).
References:
1: MSD Manual Professional Edition. 2021. Aortic Dissection – Cardiovascular Disorders – MSD Manual Professional Edition. [online] Available at: <https://www.merckmanuals.com/professional/cardiovascular-disorders/diseases-of-the-aorta-and-its-branches/aortic-dissection?query=thoracic%20aortic%20dissection> [Accessed 17 July 2021].
2: Weigang E, Nienaber CA, Rehders TC, Ince H, Vahl CF, Beyersdorf F. Management of patients with aortic dissection. Dtsch Arztebl Int. 2008;105(38):639-645. doi:10.3238/arztebl.2008.0639 [Accessed 17 July 2021]
3: Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly. 2017 Aug 25;147:w14489. doi: 10.4414/smw.2017.14489. PMID: 28871571. [Accessed 17 July 2021]
4:MSD Manual Professional Edition. 2021. Thoracic Aortic Aneurysms – Cardiovascular Disorders – MSD Manual Professional Edition. [online] Available at: <https://www.merckmanuals.com/professional/cardiovascular-disorders/diseases-of-the-aorta-and-its-branches/thoracic-aortic-aneurysms> [Accessed 17 July 2021].
5: Perlstein, I., Wang, Z., Song, Y., Wang, J., Bedford, B., Chang, M., Pursley, J., LaCreta, F., Frost, R. and Frost, C., 2014. Reversal of Apixaban Anticoagulation By 4-Factor Prothrombin Complex Concentrates in Healthy Subjects. Blood, 124(21), pp.345-345. [Accessed 17 July 2021]
6: Manea MM, Dragos D, Antonescu F, Sirbu AG, Tiron AT, Dobri AM, Tuta S. Aortic Dissection: An Easily Missed Diagnosis when Pain Doesn’t Hold the Stage. Am J Case Rep. 2019 Dec 1;20:1788-1792. doi: 10.12659/AJCR.917179. PMID: 31786581; PMCID: PMC6910182. [Accessed 17 July 2021]
7: Christie, MD, M., Brown, MD, A. and Gonzalez, MD, K., 2021. Aortic Dissection Repair Complications: ED presentations, evaluation, and management – emDOCs.net – Emergency Medicine Education. [online] emDOCs.net – Emergency Medicine Education. Available at: <http://www.emdocs.net/aortic-dissection-repair-complications-ed-presentations-evaluation-management/> [Accessed 17 July 2021].
8: Gaul, C., Dietrich, W., Friedrich, I., Sirch, J. and Erbguth, F., 2007. Neurological Symptoms in Type A Aortic Dissections. Stroke, 38(2), pp.292-297. [Accessed 17 July 2021].


This Post Has 0 Comments