So what is all the talk regarding hypercoagulability and elevated D-dimers in COVID patients?
There have been reports of VTE or hyper coagulopathic states in corona virus patients, however the clinical variability of this seems to be large given information from multiple sources. The suspicion is that a sepsis induced progressive cytokine mediated pro inflammatory condition, in conjunction with hypoxia, creates a hypercoagulable state and possibly even DIC in severe and/or progressive COVID 19 patients. D-dimers have been obtained in many different countries in conjunction with clinical management, and there seems to be some correlation with significantly elevated D-dimer and worse overall prognosis.
D-Dimer by itself is an acute phase reactant. It can be elevated in multiple conditions and is nonspecific in general, however correlated highly with clinical practice in investigations for thromboses. It depends on the purpose of obtaining the test – are we looking for a thrombosis or are we looking for a prognostic marker in COVID-19 suspected patients? Maybe they are one and the same in this current climate? We will get into that here a little further.
There have been several retrospective small trials done, primarily Chinese studies that show approximately a 19% incidence of elevated D-dimer, and in these patients mortality was high (67%) due to suspected thromboembolic complications in critically ill patients. (2,3). Unfortunately none of the studies show any data in regards to fibrinogen, PT/INR other typical markers that would be utilized to diagnose or evaluate specifically for DIC. That is not to say that these were not performed or the patients were not diagnosed with DIC, just simply that we do not have the data at this time based on the publications. So which event is increasing the mortality, and how do we effectively utilize a dimer on these patients going forward?
If I get a dimer on a COVID suspected patient, what do I do with it?
There was a Chinese study post in The Lancet on March 20th, sample size was 1008 patients. About 25 patients in the study had subsegmental PE’s, 1 patient had a DVT and 3 patients had thromboembolic strokes (1,2,3). All of these patients recieved LMWH 0.6mg/kg q12hrs and subsequently followup evaluations with CTPA – the CTPA cohort had reduced filling defects after the heparin use (less PE burden). Interestingly none of the PE’s that were demonstrated were large vessel or saddle embolus, they were all SSPE, begging the question – is this more of a microvascular phenomenon?
A Dutch study in Thrombosis Research evaluated 184 patients. They used standard VTE prophylaxis with LMWH and still had a 31% incidence of thrombosis with 25 patients having acute PE’s. Their conclusion was that a higher dose of VTE prophylaxis was likely required. (5)
The Journal of Thrombosis and Hemostasis recently published an article regarding coagulopathy in COVID patients stating the use of anticoagulant therapy with heparin was shown to decrease mortality. (1) This was especially so in patients who met sepsis induced coagulopathy (SIC) criteria ≥4 (40.0% vs 64.2%, P=0.029) compared to those with SIC score <4 (29.0% vs 22.6%, P=0.419) or with markedly elevated D‐dimer greater than six‐fold at the upper limit of normal. In pts with D-dimer >3.0ug/mL (6x upper limit of normal) anticoagulation resulted in 20% reduction in mortality (32.8% vs 52.4%, p = 0.017). (1)
TLDR; Ok so we give them anticoagulation..
Ok, so what dose of heparin should we use?
Well, what we typically use in non-renal failure patients is LMWH 1mg/kg of something like enoxaparin (Brand name Lovenox), or 10mg fondaparinux (brand name Arixtra) for things like Unstable Angina. The Chinese study suggested anything over 0.6mg/kg seemed to reduce thrombotic events, so maybe this is reasonable? At this time an exact number in unclear. The below social media post from a physician at the NHS suggests they are using typical weight based doses of thromboprophylaxis – but no data in terms of mortality or morbidity.
The ISTH has published a recommended interim protocol (7) (figure below) until more data and trials can come to fruition. They recommend that any D-Dimer result > 3x ULN at your institution should get admitted and placed on LMWH – the dose specifically isn’t specified. Abnormal aPTT and PT is not an overt contraindication. You should run DIC studies like fibrinogen and trend these if the baseline aPTT and PT are high. An absolute contraindication would be active bleeding. A postulated benefit would be that LMWH have some anti-inflammatory properties as well which may help morbidity.
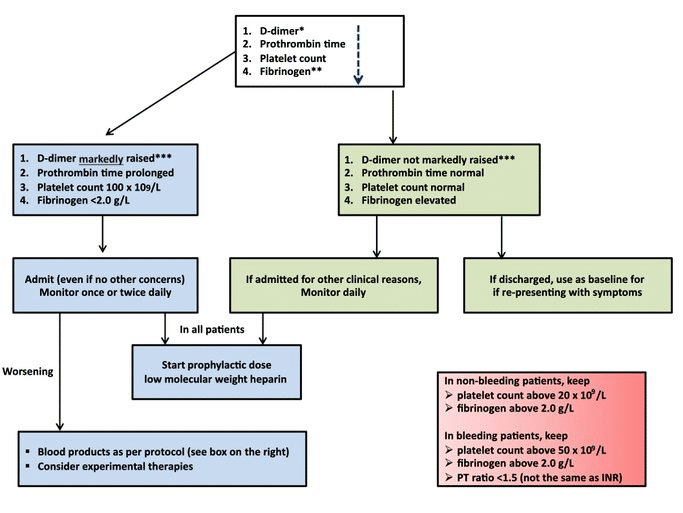
The American Society of Hematology (Link) recommends all hospitalized patients with COVID-19 receive thromboprophylaxis with LMWH or fondaparinux as opposed to UFH unless the patient is judged to be at increased risk of bleeding. They have recommended patients with a history of heparin-induced thrombocytopenia (HIT) to use fondaparinux.
What about renal failure? – I shouldn’t use LMWH for this?
Quite true – No data here to say what you should transition to, but UFH would make sense. Unfractionated heparin, argatroban, and vitamin K antagonists generally do not require dose adjustment in renal dysfunction.(11)
Ok so the Dimer is elevated and my patient is hypoxic, do I perform a CTPA or a non-con Chest CT?
Well, the data from the studies seems to suggest that the ARDS and pulmonary inflammation causes the microvascular thromboemboses – so the beginning component is the pulmonary ARDS – which can be seen easily on non-contrast Chest CT. None of the studies listed above have shown patients with massive PE, however this doesn’t mean it is an absent phenomenon. The American Society of Hematology is strongly in favor of CTPA and Bilateral Venous US of the lower extremities for PE/DVT evaluation in suspected patients in the setting of COVID (8) However if the Dimer is high, and we are planning on starting empiric heparin on these patients – should we even need a CTPA if an ECHO is normal?
Contrast – typically non-ionic – in theory poses a risk of CIN (contrast induced nephropathy) . What we do know is that CIN is quite overstated and out of date (9) so utilizing contrast shouldn’t increase too much risk. What we also know is that COVID patients can develop progressive renal dysfunction, suspected from multi-organ failure, MAHA and microvascular thromboses – should we be adding to the renal excretion burden with a contrast study?
Contrast also increases volume, and with the newest Surviving Sepsis Campaign’s recommendations to proceed with a conservative over a liberal fluid strategy (10) should we be adding more volume in general?
There are confounding variables in the setting of COVID patients with cardiomyopathy – making it hard to tell if there is massive vs submassive PE with RV/LV dysfunction if this exists, or if it is simply related to cytokine-mediated or viral induced cardiomyopathy- this hasn’t been studied yet.
TLDR; Hard to know with the recommendations the perfect choice. Depends on clinical situation. Most moderately elevated dimers with +COVID I will likely perform non-contrast chest CT and give heparins with admission. Dimer >2500 with continued hypoxia I will typically perform CTPA.
What about TPA?
In Journal of Thrombosis and Hemostasis a case series has been published which showed 3 cases of off-label intravenous administration of tPA (Alteplase) for patients with COVID-19. In all 3 cases the patients demonstrated an initial improvement in their P/F ratio, with improvements ranging from a 38% improvement to a ~100% improvement. The observed improvements were transient and lost over time in all 3 patients after completion of their tPA infusion.(12)
In a study done long ago in 1990 by Hardaway et al using fibrinolytic therapy in ARDS, they re-dosed the fibrinolytic agent subsequently in pigs with ARDS (Non- COVID) who had much more sustained responses as opposed to those observed in the JTH study (13). Hard to know how applicable that would be in COVID patients but it is interesting.
Recently published in the LA Times is an article about TPA usage. No data though, but anecdotally appears to have some results.
More research is needed.
References:
- The Journal of thrombosis and hemostasis – doi:10.1111/jth.14821
- https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2930566-3. Zhou F et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020. PMID: 32171076
- Chen J et al. Findings of Acute Pulmonary Embolism in COVID-19 Patients. Lancet 2020. [Epub Ahead of Print]
- https://emcrit.org/ibcc/COVID19/
- Klok FA et al. Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19. Thrombosis Research. [Epub Ahead of Print]
- Chen, Jianpu and Wang, Xiang and Zhang, Shutong and Liu, Bin and Wu, Xiaoqing and Wang, Yanfang and Wang, Xiaoqi and Yang, Ming and Sun, Jianqing and Xie, Yuanliang, Findings of Acute Pulmonary Embolism in COVID-19 Patients (3/1/2020). Available at SSRN: https://ssrn.com/abstract=3548771 or http://dx.doi.org/10.2139/ssrn.3548771
- Thachil J et al. ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis 2020. [Epub Ahead of Print]
- COVID-19 and pulmonary embolism https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism)
- CIN is overstated https://www.medscape.com/viewarticle/924001
- https://rebelem.com/surviving-sepsis-campaign-guidelines-on-the-management-of-critically-ill-adults-with-covid-19/
- Anticoagulant use in patients with chronic renal impairment. J.Am .Card. https://www.ncbi.nlm.nih.gov/pubmed/16156685
- Case series for TPA in COVID Journal of Thrombosis and Haemostasis 2020.https://onlinelibrary.wiley.com/doi/10.1111/jth.14828
- R. M. Hardaway et al., Prevention of adult respiratory distress syndrome with plasminogen activator in pigs. Crit Care Med 18, 1413-1418 (1990)

This Post Has 0 Comments